Congenital Heart Disease
| Congenital Heart Disease | ||
|---|---|---|
Type of Defect | Mechanism | |
| Ventricular Septal Defect (VSD) | There is a hole within the membranous or muscular portions of the intraventricular septum that produces a left-to-right shunt, more severe with larger defects | |
| Atrial Septal Defect (ASD) | A hole from a septum secundum or septum primum defect in the interatrial septum produces a modest left-to-right shunt | |
| Patent Ductus Arteriosus (PDA) | The ductus arteriosus, which normally closes soon after birth, remains open, and a left-to-right shunt develops | |
| Tetralogy of Fallot | Pulmonic stenosis results in right ventricular hypertrophy and a right-to-left shunt across a VSD, which also has an overriding aorta | |
| Transposition of Great Vessels | The aorta arises from the right ventricle and the pulmonic trunk from the left ventricle. A VSD, or ASD with PDA, is needed for extrauterine survival. There is right-to-left shunting. | |
| Truncus Arteriosus | There is incomplete separation of the aortic and pulmonary outflows, along with VSD, which allows mixing of oxygenated and deoxygenated blood and right-to-left shunting | |
| Hypoplastic Left Heart Syndrome | There are varying degrees of hypoplasia or atresia of the aortic and mitral valves, along with a small to absent left ventricular chamber | |
| Coarctation of Aorta | Either just proximal (infantile form) or just distal (adult form) to the ductus is a narrowing of the aortic lumen, leading to outflow obstruction | |
| Total Anomalous Pulmonary Venous Return (TAPVR) | The pulmonary veins do not directly connect to the left atrium, but drain into left innominate vein, coronary sinus, or some other site, leading to possible mixing of blood and right-sided overload | |

At the right is a probe patent foramen ovale in an adult. A metal probe lifts the septum secundum and reveals the opening. Normally, the left atrial pressure keeps the foramen closed, but if right atrial pressures rise with pulmonary hypertension (as with pulmonary embolus), the foramen may open and even allow a thrombus to go from right to left. This is a "paradoxical embolus", rare (seen on the left here), and so called because a thromboembolus arising from the venous circulation can end in the systemic circulation.
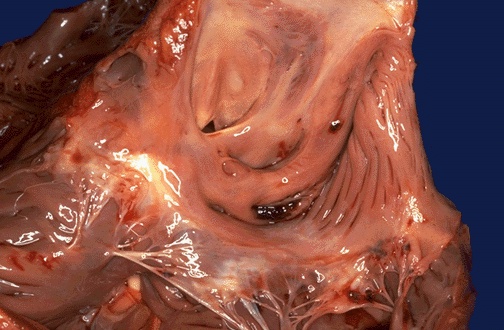
In the region of the foramen ovale on the interatrial septum is a small atrial septal defect, as seen in this heart opened on the right side. Here the defect is not closed by the septum secundum, so a shunt exists across from left to right.

This large atrial septal defect with left-to-right shunt resulted in pulmonary hypertension with increased pulmonary arterial pressures that eventually led to reversal and right-to-left shunt, resulting in marked right ventricular hypertrophy. This result from a cardiac septal defect is known as Eisenmenger's complex. The finger at the left is holding a markedly thickened right ventricular free wall below the tricuspid valve, and the finger at the right is holding the interventricular septum.

This is the heart of a premature stillborn with Trisomy 13 in which a ventricular septal defect is visible in the membranous septum. About 90% of VSD's are in the membranous septum and 10% in the muscular septum

Here is a heart with both an atrial septal defect (ASD) and a muscular ventricular septal defect (VSD). The heart is opened on the left side. Such small defects do not produce significant left-to-right shunting, but they do increase the risk for infective endocarditis.

This portion of aorta was resected from a patient with a coarctation. The aorta narrows postductally here to about a 3 mm opening.

The aorta is opened longitudinally here to reveal a coarctation. In the region of the narrowing, there was increased turbulence that led to increased atherosclerosis

This is an unusual anomaly (and not too significant for the patient).This demonstrates the power of simple observation and use of your knowledge of anatomy. Many conditions in medicine can be diagnosed by just describing what you see. How many cusps are there supposed to be on this pulmonic valve. This is a quadricuspid valve.

Here is a congenital bicuspid aortic valve. Most bicuspid valves are prone to calcification. Patients can remain relatively asymptomatic until the stenosis reaches a critical point when congestive heart failure rapidly ensues. The dense white nodules of calcification are present on either valve surface. The valve here has been opened with the aortic outflow above and the left ventricular myocardium below.

An aortic valve need not be bicuspid to calcify. Sometimes in older adults, a normal tricuspid aortic valve will undergo calcification, a so-called "senile calcific aortic stenosis." Nodules of calcification are seen on the cusps here.
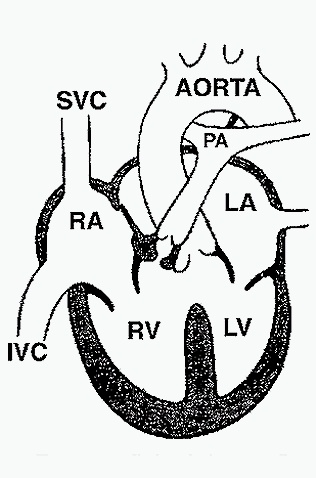
This diagram depicts the features of Tetralogy of Fallot:1. Ventricular septal defect; 2. Overriding aorta; 3. Pulmonic stenosis; 4. Right ventricular hypertrophy. The obstruction to right ventricular outflow creates a right-to-left shunt that leads to cyanosis.

The diagram above depicts the findings with a persistent truncus arteriosus. This occurs when there is failure of fusion and descent of the spiral ridges of the truncus and conus that would ordinarily divide into aorta and pulmonic trunck respectively. When the spiral septum fails to completely descend, the aortic and pulmonic trunks are left undivided at their outflow.The truncus overrides both ventricles.The persistent truncus is always accompanied by a membranous ventricular septal defect.

In the diagram above, transposition of the great vessels is shown. This occurs when the trunco-conal septum does not spiral down. Instead, it descends straight down. As a result, the outflow of right ventricle is into the aorta and the outflow from the left ventricle is into the pulmonic trunk.In order for this system to work, there must be a connection between the system and pulmonic circulations. Sometimes this is through a ventricular septal defect or an atrial septal defect. In the diagram at the left, this is through a patent ductus arteriosus.
_________________________________________________________________
Cardiomyopathies
| Cardiomyopathies | ||
|---|---|---|
| Type of CMP | Findings | |
| Dilated (Congestive) | All four chambers are dilated, and there is also hypertrophy. The most common cause is chronic alcoholism, though some may be the end-stage of remote viral myocarditis. | |
| Hypertrophic | The most common form, idiopathic hypertrophic subaortic stenosis (IHSS) results from asymmetric interventricular septal hypertrophy, resulting in left ventricular outflow obstruction. | |
| Restrictive | The myocardium is infiltrated with a material that results in impaired ventricular filling. The most common causes are amyloidosis and hemochromatosis. | |

This very large heart has a globoid shape because all of the chambers are dilated. It felt very flabby, and the myocardium was poorly contractile. This is an example of a cardiomyopathy. This term is used to denote conditions in which the myocardium functions poorly and the heart is large and dilated, but there is no specific histologic finding.

Here is a large, dilated left ventricle typical of a dilated, or congestive, cardiomyopathy. Many of these have no known etiology (so-called "idiopathic dilated cardiomyopathy") while others may be associated with chronic alcoholism. The heart is very enlarged and flabby.

Microscopically, the heart in cardiomyopathy demonstrates hypertrophy of myocardial fibers (which also have prominent dark nuclei) along with interstitial fibrosis.

There is marked left ventricular hypertrophy, with asymmetric bulging of a very large interventricular septum into the left ventricular chamber. This is hypertrophic cardiomyopathy. About half of these cases are familial, though a variety of different genes may be responsible for this disease. Both children and adults can be affected, and sudden death can occur. Seen here is the explanted heart. Pacemaker wires enter the right ventricle. The atria with venous connections, along with great vessels, remained behind to connect to the transplanted heart (provided by someone who cared enough to make transplantation possible).

Hemochromatosis, with excessive iron deposition, can occur in the heart as shown here microscopically with Prussian blue iron stain. The excessive deposition of iron leads to heart enlargement and failure similar to a cardiomyopathy, making hemochromatosis a form of "restrictive" cardiomyopathy.

This section of myocardium demonstrates amorphous deposits of pale pink material between myocardial fibers. This is characteristic for amyloid. Amyloidosis is a cause for "infiltrative" or "restrictive" cardiomyopathy. It is a nightmare for anesthesiologists when intractable arrhythmias occur during surgery on such patients

A Congo red stain has been performed on the myocardium in a case of amyloidosis. The amyloid stains orange-red, but with polarized light, the amyloid has an "apple-green" birefringence as seen here.

This left ventricle is very thickened (slightly over 2 cm in thickness), but the rest of the heart is not greatly enlarged. This is typical for hypertensive heart disease. The hypertension creates a greater pressure load on the heart to induce the hypertrophy.

The left ventricle is markedly thickened in this patient with severe hypertension that was untreated for many years. The myocardial fibers have undergone hypertrophy.
_________________________________________________________________
Miscellaneous Cardiac Diseases

The peripheral coronary arteries may undergo sclerosis, as seen here in an artery branch with a very small lumen, with chronic hypertension

The right ventricle and atrium are opened to reveal a pacemaker wire that extends to the apex to embed on the septum. Pacemakers aid in maintaining a rhythm in hearts prone to arrhythmias.

This is an excised porcine bioprosthesis; the undersurface is at the left and the outflow side is at the right. Note there are three cusps sewn into a synthetic ring. The main advantage of a bioprosthesis is the lack of need for continued anticoagulation. The drawback of this type of prosthetic heart valve is the limited lifespan, on average from 5 to 10 years (but sometimes shorter) because of wear and calcification.

This is a mechanical valve prosthesis of the more modern tilting disk variety. Such mechanical prostheses will last indefinitely from a structural standpoint, but the patient requires continuing anticoagulation because of the exposed non-biologic surfaces. The superior aspect (here the left atrium) is seen at the left, while the outflow, with the two leaflets tilted outward toward the left ventricle, is at the right in this mitral valve prosthesis.

|
